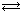 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
| Chapter Eight, Section One | |
OBJECTIVES
I. Acid and Base Strength
According to Arrhenius an acid is a substance that produces H3O+ ions in aqueous solution and a base is a substance that produces OH- ions in aqueous solution. Actually Arrhenius defined an acid as one that produces H+ ions in solution, but we know now that such ions don't exist in water:
H+(aq) + H2O(liq) --> H3O+(aq)
An example of a acid in water is HCl, a very strong acid
HCl + H2O --> H3O+ + Cl-
Some bases such as NaOH are solid ionic compounds and the ions are separated when the compound is dissolved in water, so that the OH- ion is produced in solution:
NaOH(s) + H2O --> Na+(aq) + OH-(aq) + H2O(liq)
Other bases are not hydroxides, but do produce OH- ions in solution:
NH3(aq) + H2O 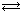 NH4+(aq) + OH-(aq)
NH4+(aq) + OH-(aq)
The above reaction is shifted more toward the left, producing only a small amount of hydroxide ion.
II. Acid and Base Strength
All acids and bases are not equally "strong". The degree of the strength relates to the amount that the water reaction of the acids and bases with water is shifted to the product side. HCl, HI, HBr, HNO3, H2SO4, and HClO4 are strong acids and NaOH, KOH, LiOH, and Ba(OH)2 are strong bases. Almost all other acids and bases are weak. Acetic acid is a weak acid:
CH3COOH(aq) 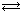 CH3COO-(aq) + H3O+(aq)
CH3COO-(aq) + H3O+(aq)
where the reaction is shifted much more to the left than to the right. It is a good thing that acetic acid is a weak acid or some of the salad dressings would burn up your lettuce!
II. Bronsted-Lowrey Acids and Bases
Bronsted and Lowrey developed another definition for acids and bases to account for situations in which water is not involved: an acid is a proton donor and a base is a proton acceptor. Note the following example for a couple of new concepts:
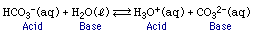
One new concept is that an acid or a base can be either a molecule or an ion. Note that water is the base because it accepts a proton and H3O+(aq) is an acid because it can give up a proton. Cl- is a base because it can accept a proton, and HCl is an acid because it donated a proton. H3O+(aq) is called the conjugate acid of the base H2O and CO32- is the conjugate base of the acid HCO3-. This acid, HCO3-, gave up only one proton so it is called monoprotic. Other acids, such as H2SO4, are capable of giving up more than one proton, and it would be called diprotic. However such acids that can give up more than one proton do so sequentially in monoprotic steps:
H2SO4 + H2O --> HSO4- + H3O+
HSO4- + H2O --> SO42- + H3O+.
Be sure to study Table 8.2 in your book, which shows several acids and their conjugate bases.
III. Acid Dissociation Constants
In chapter 7 we learned about equilibrium constants. Now we can apply that concept to weak acids in solution to inform us as to how strong a weak acid is. A weak acid added to water gives us the equilibrium:
HA(aq) + H2O(liq) 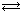 H3O+(aq) + A-
H3O+(aq) + A-
For this reaction the equilibrium constant is
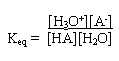
However the concentration of water in the denominator does not vary much so we can multiply both sides by [H2O] to give a new equilibrium constant, Ka, called the acid dissociation constant:
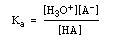
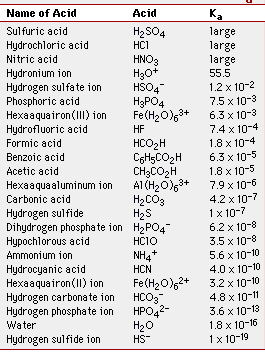
Below is a table of Ka values for some common weak acids. The smaller the Ka, the weaker the acid, or the less amount of H3O+ produced.
A similar situation holds for weak bases:
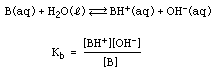
And we have a similar table for the weak bases:
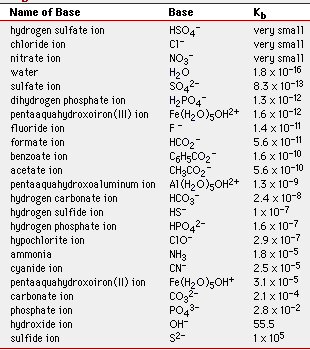
The smaller the Kb value, the weaker the base.
IV. Properties of Acids and Bases
The most important property of acids and bases is that they react with each other in a process called neutralization. A strong acid and a strong base will react with each other to produce a salt and water:
HCl + NaOH --> NaCl + H2O
V. Reactions of Acids
Acids react with active metals to produce hydrogen and a salt:
Mg(s) + 2 HCl --> MgCl2(aq) + H2(g)
Acids react with metal hydroxides to give water and a salt:
HNO3 + KOH --> H2O + KNO3
Strong acids react with metal oxides to produce water and the metal:
2 H3O+(aq) + CaO(s) --> 2H2O(liq) + Ca2+
Strong acids added to a carbonate or a bicarbonate give off bubbles of carbon dioxide:
H3O+(aq) + CO3-(aq) --> H2CO3(aq) + 2 h2(liq)
H2CO3(aq) --> CO2(g) + H2
Finally, any acid stronger than NH4+ will react with ammonia the same as HCl will:
HCl + NH3 -- > NH4+ + Cl-
After you have studied this material and practiced some problems, take quiz one. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs - ALL RIGHTS RESERVED