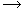 H3O+ + OH-
H3O+ + OH-
| Chapter Eight, Section Two | |
OBJECTIVES
I. Self-Ionization of Water
Perhaps surprisingly, pure water does contain a small amount of H3+ and OH- ions.
H2O + H2O 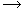 H3O+ + OH-
H3O+ + OH-
Writing the equilibrium constant and then multiplying both sides by [H2O]2 gives
Keq[H2O]2 = [H3O+][OH-]
Since the concentration of water is virtually constant, we can replace the left hand side with a new equilibrium constant, Kw, called the ion product of water:
Kw = [H3O+][OH-]
At room temperature, Kw = 1.0 x 10-14. The autoionization of water produces both the ions so their concentrations must be equal, or
[H3O+] = 1 x 10-7
[OH-] = 1 x 10-7
Kw = [H3O+][OH-] applies to any water solution. However, the individual concentration of [H3O+] and OH- will change, but their product will always be 1 x 10-14. Very important. Make sure that you understand this concept by working problem 8.2 on page 207.
II. pH, pOH, and pK
The p's are introduced for ease of communication of information about acid/base systems. One way to think about it is that the "p" stands for "-log[]". So pH = -log[H3+], pOH = -log[OH-], and pK = -logK. Remember that Kw = 1.0 x 10-14, so that -logKw = 14. But since Kw = [H3O+][OH-], AND since the log of a product of things is the sum of the logs {log(ab) = log(a) + log(b)}, we have that
14 = pH + pOH
Then if we know the pH we can easily calculate the pOH, or vice versa.
Acid solutions have a pH less than 7 and a basic solution has a pH greater than 7. The lower the pH, the more acidic the solution and the higher the pH, the more basic the solution. It is also important to understand that a change of one in the pH value means a ten-fold change in the [H3+], which is considerable. We can obtain qualitative measures of the pH by using either pH paper or an indicator.
Some common indicators and the pH range over which they change color are:
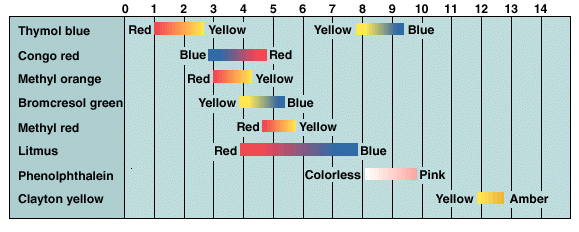
We can obtain quantitative measures of the pH by using a pH meter as indicated in Figure 8.5 on page 211.
III. pH of aquous solutions of salts
Salts will be soluble in water, but will any of the resultant ions react with water? If one of the ions reacts with water and produces [H3O+] then the resultant solution is acidic. If one of the ions reacts with water and produces [OH-] then the resultant solution is basic. So we just need to know what happens when the salt dissociates in water and whether the resultant solution is acidic or basic will then be determined. We have four possibilities
You have to then refer to our previous discussions to determine what sort of acid and base components of the salt we have.
After you have studied this material and practiced some problems, take quiz two. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs - ALL RIGHTS RESERVED