 C + D
C + D
| Chapter Seven, Section One | |
OBJECTIVES
I. Introduction and Collision Factors
A. Generalities
Some reactions occur very rapidly, some reactions occur so slowly that it is difficult to determine within our lifetime if any product is being formed, and of course many reactions occur at rates in between these limits. An example of a very slow reaction may surprise you:
2 H2 + O2 --> 2 H2O
Just putting the two gases together in a container and measuring for the formation of water, one can wait several years and not discern that any water has been formed!
Chemical kinetics is the study of the rates of chemical reactions. The rate of a chemical reaction is defined as "the change in the concentration of a reactant or product per unit time". These rates must be experimentally determined. We can write them by considering the following hypothetical reaction:
A + B  C + D
C + D
We can look at either the time rate of loss of A, the time rate of loss of B, the time rate of formation of C, or the time rate of formation of D. We will let  stand for the change in concentration of a
reactant or product, so the time rate of formation of C would be
stand for the change in concentration of a
reactant or product, so the time rate of formation of C would be
 C/
C/ t
t
where t is the time. With all stoichiometric coefficients being equal, the rates of formation and loss would be all equal:
 C/
C/ t =
t =  D/
D/ t = -
t = - A/
A/ t = -
t = - B/
B/ t
t
Note the minus signs for A and B since they are being lost.
To get numbers for these expressions we would have to measure the loss of A during a particular time period and then perform the division (note the example in your book).
B. Molecular Collisions, Activation Energy, and Energy Diagrams
No collision, no reaction! That should not come as a big surprise! However, just because a collision occurs does not mean that a reaction will occur between the two colliders -- that may be a surprise. Two details about a collision are important for a reaction to occur:
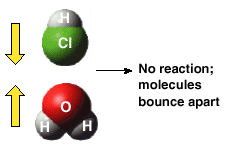
In the following, the two molecules are oriented favorabley for a reaction to occur:
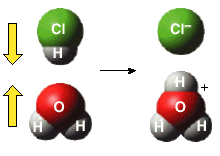
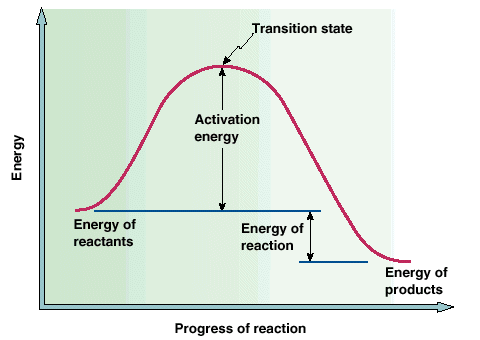
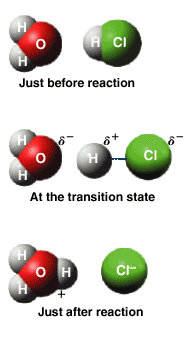
The transition state is a state that exists for only a small amount of time during which the reactants are "coming apart" and the product is being formed as indicated below.
II. Factors Affecting Rates of Reactions
A. Concentration
For the general reaction: A + B  C + D, if we double the amount of A and double the amount of B we would expect the rate of the reaction to increase because we have doubled the possible
number of collisions. (We will talk more about this in the next section.) We can write the rate of the reaction as
C + D, if we double the amount of A and double the amount of B we would expect the rate of the reaction to increase because we have doubled the possible
number of collisions. (We will talk more about this in the next section.) We can write the rate of the reaction as
rate = k[A]
where k is the rate constant, and the brackets around A indicate the concentration of A. Here you see a direct dependence of concentration on rate. This can get rather complicated, but we will keep it simple. Now if the rate has been determined like in the previous section, we can solve for the rate constant: k = rate/[A]. Study the example in your book.
B. Temperature
We will study this more in the next section, but if you increase the temperature of the reaction, you would probably expect the rate to increase for you have increased the number of collisions per second and you have increased the collision energy (all other things being the same). This is true for an endothermic reaction, and a general rule is that for such reactions as the temperature increases 10oC, the rate doubles.
C. Catalyst
A positive catalyst (one that increases the rate of a reaction) functions by lowering the activation energy of the reaction as indicated below:
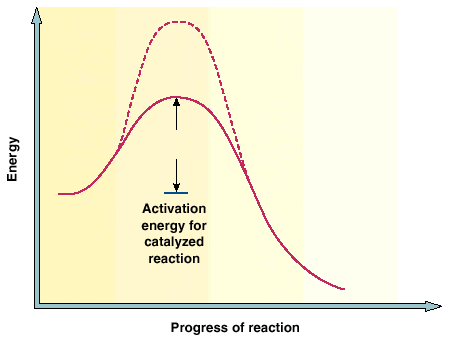
The definition of a catalyst is generally stated as "any substance that increases the rate of a chemical reaction without itself being used up in the reaction". Exactly how the catalyst allows the reaction to proceed via a path with a lower activation energy can be rather complicated and depends upon the particular reaction. You can study this in more advanced chemistry courses, and it can be very interesting.
The following web sites may help you understand some of the concepts. Some of the discussions on the sites are a little above the level of this course, but do take a look at them.
After you have studied this material and practiced some problems, take quiz one. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs - ALL RIGHTS RESERVED