 C + D,
C + D,
| Chapter Seven, Section Two | |
OBJECTIVES
I. Reversible Reactions and Equilibrium
In writing the hypothetical reaction,
A + B  C + D,
C + D,
we seem to be assuming that the reverse of the reaction cannot occur. An irreversible reaction is one that can only occur in one direction. If we had written the reaction as
A + B  C + D,
C + D,
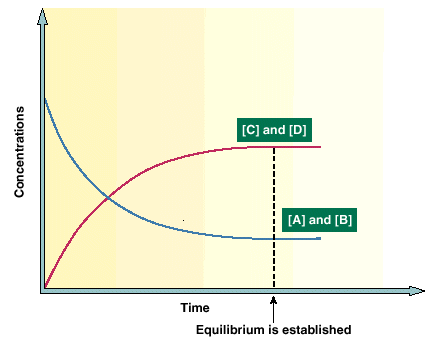
then you might assume that the reaction can go in either direction. A reversible reaction is one that can be made to go in either direction. It is rare that a reaction will go to completion, meaning that there are no reactants left. The usual fate of a chemical reaction is that after some time has elapsed the reaction will be in dynamic equilibrium, meaning that the same A and B molecules are not always present but the same number of A and B molecules are present. Thus after dynamic equilibrium is established the concentrations of A, B, C, and D do not change. This is indicated in the following diagram.
Read the material in your book.
II. Equilibrium Constants
Let's consider the hypothetical reaction
aA + bB + ....  cC + dD + ....
cC + dD + ....
where the "...." means that more molecules could be present. The expression for the equilibrium constant, K, for the reaction is then given as
K = [C]c[D]d....÷ ([A]a[B]b...)
Study the material in your book and be sure that you can write expressions for the equilibrium constant for any reaction. The equilibrium constant is truly a constant unless the temperature is changed. We can indicate the temperature dependence of K by writing K(T). If K is very small, then there is not much product formed. If K is very large then much product has been formed. These last two statements should be obvious from just looking at the form of K above. Remember that the equilibrium expression is only valid after equilibrium has been established.
III. Equilibrium and Reaction Rates
There is NO relationship between K and the rate of a reaction. It is possible to have a large K and a low rate, or a small K and a high rate. We cannot infer anything about the rate of a reaction from the equilibrium constant, these are two separate concepts.
IV. Le Chatelier Principle
Le Chatelier's Principle is: if an external stress is applied to a system in equilibrium, the system reacts in such a way as to partially relieve the stress. There are four possible ways that stress can be applied to a reaction.
 C + D. After equilibrium is established, if we add more A then the reaction will
shift to produce more C and D (assuming we have enough B to accomplish this feat). If we add more C, the reaction will shift toward the reactant side to try to remove the stress we imposed upon it.
C + D. After equilibrium is established, if we add more A then the reaction will
shift to produce more C and D (assuming we have enough B to accomplish this feat). If we add more C, the reaction will shift toward the reactant side to try to remove the stress we imposed upon it.
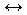 C + D. If, after equilibrium is established, we remove some A then the reaction will shift to
the left to reestablish equilibrium. If we remove some C then the reaction will shift to the right to make some more C to reestablish equilibrium.
C + D. If, after equilibrium is established, we remove some A then the reaction will shift to
the left to reestablish equilibrium. If we remove some C then the reaction will shift to the right to make some more C to reestablish equilibrium.
A + B 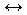 C + D + 15,000 cal
C + D + 15,000 cal
A + B + 15,000 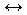 C + D
C + D
The first reaction is an exothermic reaction and if we add heat (adding "product") the equilibrium will shift to the left to try to relieve the stress. The second reaction is an endothermic reaction and if we add heat to it (adding "reactant") the equilibrium will shift to the right to try to relieve the stress. You should be able to understand what happens to each when we remove heat from the reactions.
After you have studied this material and practiced some problems, take quiz two. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs - ALL RIGHTS RESERVED