
| Chapter Five, Section Three | |
OBJECTIVES
I. Evaporation and Condensation and Boiling Point
All liquids will evaporate if left in an open container. This process is called evaporation and it occurs when the heat brings the liquid molecules to the gaseous state (giving them enough kinetic energy so that they can break free of the intermolecular forces holding them in the liquid state). The stronger the intermolecular forces the greater the heat required for evaporation to occur. This is a dynamic situation in which some molecules will collide with each other and lose energy and return to the liquid state (condensation). If the liquid is in a closed container, the situation will reach equilibrium in which there are the same number of molecules leaving the liquid as there are returning to the liquid. The pressure of the gas molecules is called the vapor pressure. The vapor pressure is obviously a function of temperature (obvious to you?). The stronger the intermolecular forces the lower the vapor pressure at some particular temperature (obvious?).
As the temperature increases, the vapor pressure increases. The definition of boiling point is the temperature at which the vapor pressure of the liquid is equal to the pressure of the atmosphere in contact with the liquid. When the vapor pressure reaches 1 atmosphere the temperature is called the normal boiling point (100 o for water at sea level). Since the atmospheric pressure decreases as elevation increases, the boiling point of water will decrease as we go higher in elevation. So if you are camping in the Rockies, you will have to boil your egg longer than at sea level to get the same degree of cooking (why?).
Now remember the previous discussion on intermolecular forces. Remember that dispersion interactions increase as the size of the molecule increases. So we would expect the boiling point of CH3CH2CH2CH2CH3 to be greater than that of CH3CH2CH3. Experiment indeed verifies our deduction.
II. Solids
The solid state is characterized by a very regular arrangement of atoms or ions in a crystal lattice, such as NaCl as we had looked at earlier:

The atoms or ions are not moving around as in the liquid or gas state (don't tell anyone, but they do vibrate about their positions). The process of obtaining a crystal from the liquid state is called crystallization. Some compounds can exist in different solid state forms. A good example is carbon which can exist in five different solid forms: graphite, diamond, soot, buckeyball, and nanotubes. Soot is an amorphous solid meaning that its atoms are not arranged in a regular array such as NaCl above. Diamond is actually one gigantic molecule called a network solid or network crystal and such crystals are extremely hard and resistant to heat.
III. Phase Changes
There are three phases of matter with which we will be concerned: solid, liquid, and gas. The heat required to change from one phase to another is the heat of the phase transition (change). So the heat required to melt a solid is the heat of fusion, the heat required to change a solid to a gas is the heat of sublimation, and the heat required to change a liquid into a gas is the heat of vaporization. We can show all of the phases on a single diagram called a phase diagram as indicated below for water and carbon dioxide.
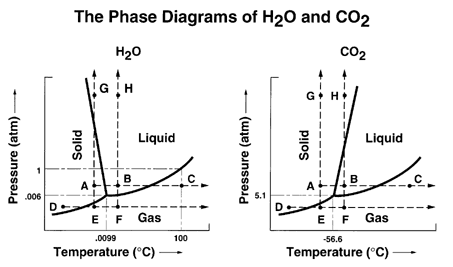
The 1 atmosphere line shows the normal melting and boiling points for water. Note that the slope of the solid-liquid line is negative for water and positive for carbon dioxide. Water is one of only a few substances which have such a negative slope and that gives water some strange properties.
Here are some good web sites to visit:
After you have studied this material and practiced some problems, take quiz three. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs - ALL RIGHTS RESERVED