OBJECTIVES:
I. IUPAC System of Nomenclature
The IUPAC system rules that you learned previously only have to be slightly modified for other classes of organic compounds. This makes it easy to tell the type of structure one has by simply looking at the IUPAC name. The suffixes tell you the type (class) of compound, the infix tells you whether there are double or triple bonds present, and the prefix tells you the number of carbons in the parent chain. Look at the examples in Section 11.6 of your text.
II. SHAPES OF CYCLIC COMPOUNDS AND STEREOISOMERISM
There are many different conformations of alkanes. You saw previously that you can draw the chain in many ways on your paper. You can stretch it from left to right, stretch it vertically, make it zig-zag, etc. These conformations occur because there is rotation around the single bonds. For cycloalkanes, however, rotation is restricted because all the carbons are joined together in a ring. For cyclopentane and cyclohexane, there are different names given to the different types of conformations. You do not need to know these names. But take a look at the pictures (Figs. 11.3 - 11.5). The most stable conformation of these is not a flat ring-even though we will continue to draw it as such because it's easier! That's all you really need to know.
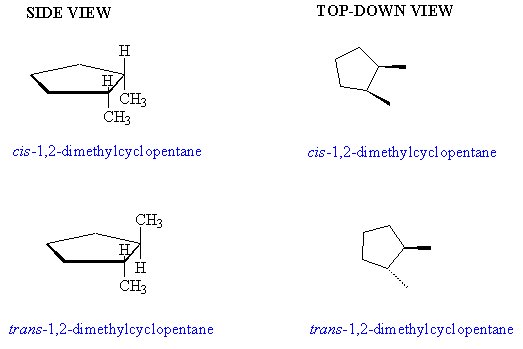
Consider the compound 1,2-dimethylcyclopentane as shown below:

[In the structures on the right, I have left out all the hydrogen atoms on the rings. The bold wedge indicates that the group is pointing up from the plane of the ring, and the broken wedge indicates that the group is pointing down from the plane of the ring.]
You see that both of these compounds are C7H14, but the structure that I call cis has both methyl groups on the same side of "plane" of the ring and the trans structure has the methyl groups on opposite sides of the plane of the ring.
If you look carefully at the structures, you will see that they are not the same. There is no way to rotate the structures around to get them to superimpose on each other. Or use your model kit to build a model and convince yourself of this. Such compounds are called stereoisomers - compounds that have the same molecular formula and the same order of attachment of atoms, but differ in how the substituted groups are oriented in space. The use of cis indicates that both groups are pointing in the same direction in space, and trans indicates that the groups are pointing in opposite directions in space. Thus, the complete names are cis-1,2-dimethylcyclopentane and trans-1,2-dimethylcyclopentane. Stereoisomers are found in rings of all sizes when there are 2 or more substituents on the ring, and they always have slightly different physical and chemical properties.
III. PHYSICAL PROPERTIES
Here we are concerned with boiling point, melting point, solubility, and density.
A. Boiling/Melting Points.
Because there is very little electronegativity difference between a carbon and a hydrogen, alkanes are nonpolar compounds. This means that the interactions between two hydrocarbons can only be due to London dispersion forces which are weak. Thus, small alkanes (up to about 5 carbons) are not very attracted to each other and are gases at room temperature. Alkanes from 5 - 17 carbons are liquids, and larger alkanes are white, waxy solids, like parrafin. As more carbon atoms are added, the number of London dispersion forces increases and that causes the molecules to be more "attracted" to each other, or to pack more closely together and exist as liquids or solids. Notice the increase in m.p. and b.p. as the carbon number increases for the unbranched alkanes in Table 11.4.
Alkanes that are constitutional isomers have different properties. Notice how the very branched 2,2-dimethylbutane has a lower boiling point than the straight-chain hexane (Table 11.5). This can also be attributed to differences in London dispersion force attractions. Spherical molecules have a lower surface area and fewer LDF attractions than do straight chain molecules.
B. Other Physical Properties
Alkanes are colorless and odorless and tasteless. They are insoluble in water. (Go back to your review sections if you're unsure why they are insoluble in water.) They are less dense than water, so if you put an alkane in water it will float to the top forming a layer.
IV. CHEMICAL PROPERTIES & SOURCES OF ALKANES
Alkanes can undergo combustion - a reaction with oxygen generally started by a flame. You may be familiar with the combustion of propane, butane, methane (a component of natural gas) or octane (a component of gasoline). This is an oxidation reaction.
C3H8 + 5 O2 --> 3 CO2 + 4 H2O + heat
Combustion is the most important reaction of alkanes. In fact, it's the only one we'll study! Study Section 11.11 to understand the real source of these gases.
After you have studied this material and practiced some problems, take quiz three. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs & Dr. Jennifer Powers & Dr. Vicky Bevilacqua - ALL RIGHTS RESERVED