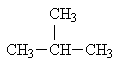
| Chapter Eleven, Section One | |
OBJECTIVES
I. Hydrocarbons
This is a really tough section. Hydrocarbons are compounds that contain only hydrogen and carbon.
Hydrocarbons with only single bonds are alkanes or saturated hydrocarbons.
Hydrocarbons with C=C bonds are alkenes.
Hydrocarbons with carbon-carbon triple bonds are alkynes.
All that we will study now are called aliphatic hydrocarbons. They have different properties than the aromatic hydrocarbons that we will study later.
That's all, folks!!
II. Alkanes
We can write the alkanes in a condensed structural formula that is easier to do over the web:
We write
Methane as CH4
Ethane as CH3 - CH3 or CH3CH3
Propane as CH3 - CH2 - CH3 or CH3CH2CH3
Note that each carbon has four bonds and each hydrogen has only 1 bond. Of course this way of writing them does not convey any of the three-dimensional nature of the molecules. I again refer you to my chime site to look at some three dimensional molecule representations. Also your book conveys the three-dimensional structure on a two-dimensional page.
Note the names as the number of carbon atoms increases: methane, ethane, propane, butane, pentane, hexane, heptane, octane, nonane, and decane. Also note that as the size increases the boiling and melting points increase because the the total attractive intermolecular forces increase.
Too easy, so let's complicate the situation some. The structures so far are all "straight-chain" or "normal" hydrocarbons. The structures can also be "non-normal" (I just made up that word) or with "branched" chains.
An example is isobutane: 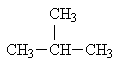
You see that the molecular formula for this is C4H10, the same as for butane. However it is not the same compound: it has different chemical and physical properties than butane. Butane and isobutane are constitutional (structural) isomers. Also notice that one carbon atom in isobutane is bonded to three different carbon atoms -- it is said to be a tertiary carbon atom. A carbon atom bonded to only one other carbon atom is called a primary carbon atom. A carbon atom bonded to two different carbon atoms is called a secondary carbon atom, and a carbon atom bonded to four different carbon atoms is called a quaternary carbon atom.
Methane has, of course, no isomers. Ethane also has no isomers. Propane also cannot have any isomers. Butane has two isomers (the normal structure and isobutane). Pentane has three isomers; the normal pentane and the two following compounds:
isopentane: 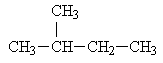
neopentane: 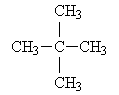
Hexane has five isomers including the normal hexane. The other four isomers are below:
2-methylpentane 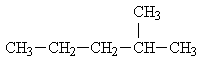
3-methylpentane 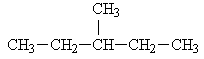
2,3-dimethylbutane 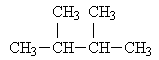
2,2-dimethylbutane 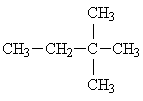
Don't worry about the names YET. The point now is that all of these isomers have the same molecular structure, but different chemical and physical properties.
To really try to confuse you, we can also draw these structures in different ways but without changing the molecule. The way to determine if two drawings are equivalent is to count down the longest continual chain, even if it twists some, and see if the number of carbon atoms in this chain is the same in both structures. Then note where the substituents are located on this chain and see that there locations are the same on both structures. Your book gives you some example compounds with which to practice.
After you have studied this material and practiced some problems, take quiz one. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs & Dr. Jennifer Powers & Dr. Vicky Bevilacqua - ALL RIGHTS RESERVED