
The alcohol methanol is CH3OH, where one of the hydrogens in methane has been replaced with a OH group. This -OH group is called the hydroxyl group and is one of many functional groups. They are called functional groups because they add functionality (the ability to act) to the molecule. Functional groups can be made of any combinations of N, O, S, H, halogens, and C atoms. Some of the most important functional groups are given in your book and you need to be familiar with them. We will here mention a few of them. Another alcohol is ethanol, shown below.
Ethanol 
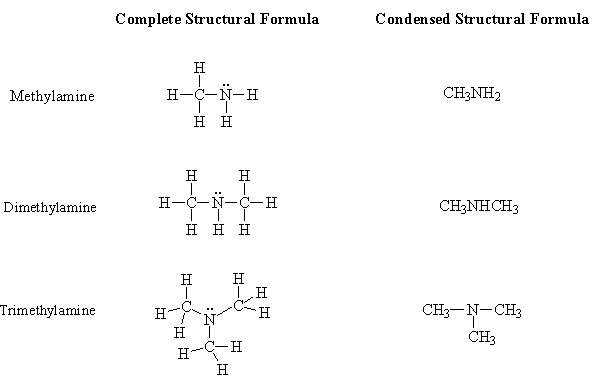
Amines
The functional group of the amine is an amino group consisting of a nitrogen atom bonded to one (primary amine), two (secondary amine) or three (tertiary amine) carbon atoms. Remember that the nitrogen atom has to have a total of three bonds. Examples of the three categories of amines are shown below:
Aldehydes and Ketones
The functional group for both aldehydes and ketones is the carbonyl group (C=O). A ketone has the carbonyl group bonded through its carbon atom to two other carbon atoms. An aldehyde has the carbonyl group bonded through its carbon atom to one other carbon atom (or in the case of formaldehyde to a carbon atom and a hydrogen atom). Remember that a carbon atom always needs a total of four bonds. Examples of aldehydes and ketones are shown below:
acetaldehyde 
acetone 
Carboxylic acids
The functional group for a carboxylic acid is a carbonyl group (-COOH), this group has one oxygen double bonded to the carbon atom and the OH group then bonded to the carbon atom. Remember that the oxygen atom needs two bonds total to it. Below is an example of carboxylic acids:
acetic acid 
Ethers
The functional group for ethers is -O- where the oxygen is bonded on both sides to a carbon atom. An example of an ether is noted below:
dimethylether 
Other Functional Groups
Now that you have an idea of what functional groups are, study the first 3 columns in the Table "Some Important Organic Functional Groups" in the back of your textbook. You will need to know the first two columns of this table for your first test.
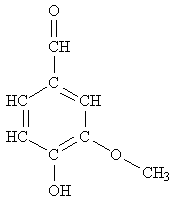
Here is an interesting molecule with a nice odor and several functional groups:
After you have studied this material and practiced some problems, take quiz two. If you score at least 80 on the test then you are ready to continue to the next section.


Web Author: Dr. Leon L. Combs
Copyright ©2001 by Dr. Leon L. Combs & Dr. Jennifer Powers & Dr. Vicky Bevilacqua - ALL RIGHTS RESERVED