Some mysteries begin to surface when we examine closely the bonding in molecules. For example, the electron configuration of carbon is
1s22s22p2
If we think about the bonding of carbon with hydrogen atoms which have the configuration
1s1
then we would think that perhaps two hydrogen atoms would combine with the two unpaired electrons in carbon to form
CH2
But carbon forms four bonds. The molecule formed by carbon combining with hydrogen is CH4. If you have the Chime plugin, go to the methane chime site. How does this bonding occur since there are only two unpaired electrons in the ground state of carbon?
Using the Lewis structure method, we drew the CH4 structure and using the VSEPR model we predicted a tetrahedral structure (we did not worry about unpairing the 2s electrons) which is what is observed experimentally. However 2s and 2p electrons have different orbital probability density diagrams so the bonds would not all be the same type, however experiment says that they are all the same. ??
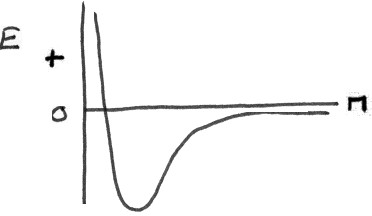
The above considerations require a new aspect to our model. We need some new types of orbitals to agree with the experimental observations so the concept of hybridization was introduced. You know what the English word hybrid means so we just apply it to our atomic orbitals. It is reasoned that the atoms will combine in such a way as to achieve the lowest energy for the resulting structure as noted in our energy diagram.

If that means forming a new set of orbitals from the original atomic orbitals, then such an approach might be reasonable. So how do we get four equivalent orbitals from the original 2s22p2 orbitals? We can take the 2s and the 2p
orbitals and form four equivalent orbitals and populate them as shown below:
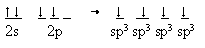
Now we have four equivalent orbitals ready to share an electron with each hydrogen atom to form the CH4 molecule. We call these the sp3 hybrid orbitals.
We can take the same approach to explain the NH3 molecule which also requires a tetrahedral structure for all electron pairs (including on lone-pair). So again we form the sp3 orbitals from the 2s and 2p orbitals and then populate them with the 5 valence electrons that nitrogen has. That arrangement will then be represented as follows:
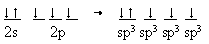
Now we are ready for three hydrogens atoms to be bonded to three of the sp3 orbitals and the other hybrid orbital is occupied by the lone pair.
I am still working on this, but some good help sites are given below.
This one is so good that I may not work on my site at all! Valence Bond Theory and Hybrid Orbitals
 Here is a site to help with the hybrid orbital concept. Here is a second site to help with the hybrid orbital concept.
Here is a site to help with the hybrid orbital concept. Here is a second site to help with the hybrid orbital concept.
 Here are some good pictures of the hybrid orbitals.
Here are some good pictures of the hybrid orbitals.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |
