
The Lewis Structures are produced assuming that atoms want to obtain a noble gas structure in their bonding environment. To make a molecule we start with an "electron dot diagram" for each atom involved in the bonding and using only the valence electrons. Then we bring the atoms together in such a way as to try to achieve a noble gas structure for each atom involved in the bonding. For a simple example consider the hydrogen molecule being made from the atoms:
H. + H. ---> H:H
So you see we have achieved the He noble gas structure around each hydrogen atom. How about F2? See it below:

Here we see that the Ne noble gas structure has been attained by each F atom. I have circled the lone pair electrons in the H-F molecule on the right. The single electron pair in the center is the bonding pair. You see that one bonding pair is a single bond.
In making more complex molecules we need to consider some simple rules such as a hydrogen atom can have only one single bond to it, a carbon atom can have a total of only 4 bonds, an oxygen atom can have only two bonds, halogens can only have one bond, and nitrogen can have only 3 bonds. When you have several atoms involved, it is usually the atom with the lowest electronegativity which goes in the center. These rules will allow you to construct a lot of molecules. For example, consider HCN. Since the hydrogen can have only one bond it will have to go on an end. The carbon atom has a lower electronegativity than N so it goes in the middle. Then the molecule must be:
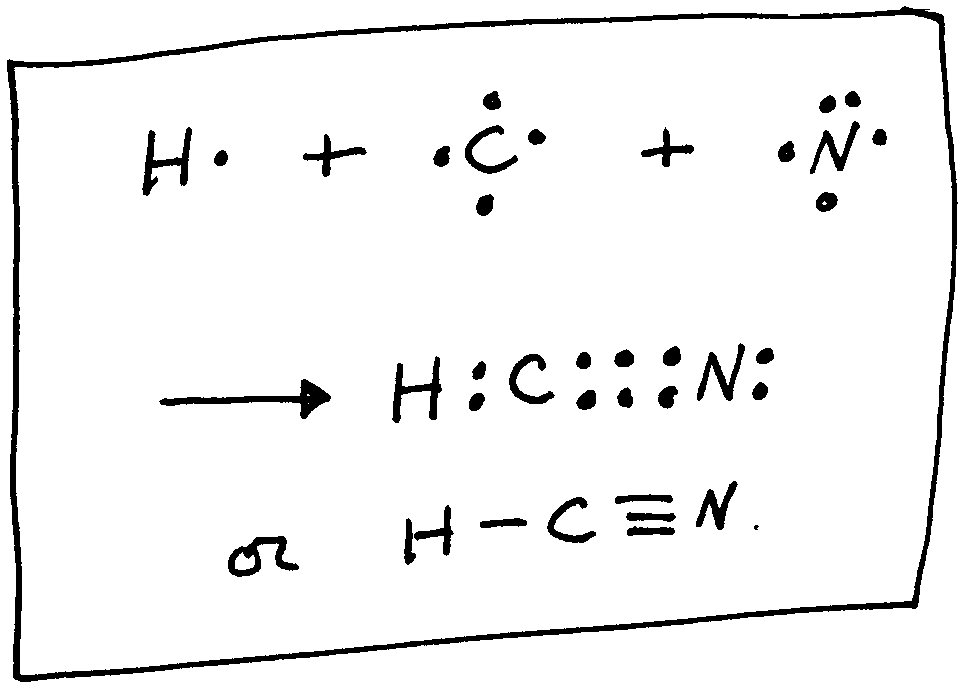
Here we see that all the atoms have achieved a noble gas structure and each have the number of total bonds that they must have (H,one; C,four; N,three). Now we have introduced a triple bond between the C atom and the N atom.
 Here is an excellent site for further study on Lewis structures. You need the plug-in Chime to do all of this site,
but not the part just dealing with Lewis structures. Click on the Lewis Structures menu after scrolling down just a little on this introductory page.
Here is an excellent site for further study on Lewis structures. You need the plug-in Chime to do all of this site,
but not the part just dealing with Lewis structures. Click on the Lewis Structures menu after scrolling down just a little on this introductory page.
Failure of the Octet Rule
If the total number of electrons is odd, there must be
For example, consider NO2. Two oxygens gives 12 valence electrons and 1 nitrogen gives 5 valence electrons for a total of 17 valence electrons. Since this is an odd number, we know that there will be a failure of the octet rule. One possible structure is
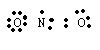
Here we see that the nitrogen has one unpaired electron which would predict that this molecule is paramagnetic and in fact it is. We could just as well have put the double bond on the left O-N pair so we would need a resonance picture for NO2 in this model.
Another example is NO which has a total of 11 valence electrons. The structure for it would be
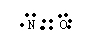
Again we see that nitrogen has an unpaired electron with the prediction that NO will be paramagnetic which indeed it is. We expect odd-electron species to be paramagnetic and even-electron species to be diamagnetic.
Just when you get used to a little success, guess what happens? Yup, failure. The O2 molecule has 12 valence electrons but it is paramagnetic!! So O2 must have at least one unpaired electron and how do we manage that? We can draw a nice Lewis structure which satisfies the octet rule as shown below, but it must be incorrect.
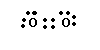
So we have to construct a Lewis structure which has unpaired electrons. We can do that with three resonance hybrids as shown below.
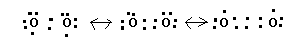
Some molecules will contain atoms with incomplete octets but an even number of electrons, such as BF3. It has 24 valence electrons but we can't draw a Lewis structure with all atoms haveing complete octets. An acceptable structure would be the on shown below.
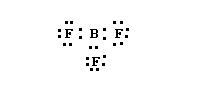
Note that the B has an incomplete octet which would imply, in this model, that it could easily react with something which could donate a pair of electrons. We could also draw 3 resonance structures for BF3 by alternating the placement of the doubel bond between each F-B pair. This resonance structure would probably be a better representation due to the experimental bond lengths having some double bond character.
Some molecules contain atoms with expanded octets. Some examples are (the first is a good little girl and obeys the rules):
| molecule | number of electrons about central atom |
| PCl3 | 8 |
| PCl5 | 10 |
| ICl4- | 12 |
| SF6 | 12 |
| I3- | 10 |
Be sure that you can draw the above and obtain the same number of electrons about the central atom.
Do remember that these are models, and crude models at that. However they are useful for an introduction to molecular bonding and shape.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |

Web Author: Dr. Leon L. Combs
Copyright ©1999 by Dr. Leon L. Combs - ALL RIGHTS RESERVED