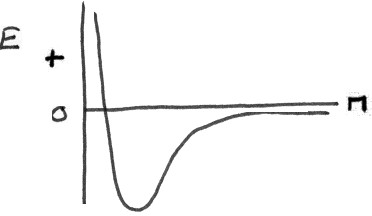

But now at the right are the parts of the molecule (whatever they are), and the lowest energy is the energy of the aggregate (the molecule). If we call this the stabilization energy then we can use it to obtain some rough values for the bond energies.
Methane (CH4) has four C-H bonds so we assume they are all of equal energy we can take the stabilization energy of methane (1652 kJ/mol) and divide by four to obtain the bond energy of each C-H bond which gives
EC-H = 413 kJ/mol.
Now we can obtain the stabilization energy of CH3Cl which is 1578 kJ/mol and determine the bond energy of the C-Cl bond if we assume that the C-H bonds in this molecule each have the same energy as each does in methane:
3*413 + EC-Cl = 1578 kJ/mol or EC-Cl = 339 kJ/mol
Now it is not really true that each C-H bond has the same energy regardless of the compound in which that bond is found because different atoms attached in the molecule will cause different electron density shifts either away or from the C-H bond and thus change it's energy. However we will make this assumption to build a model from which we can get a table of values of average bond energies (D) as shown in Table 9.9 of the Kotz book. From these values we can now estimate the deltaH values of reactions. Consider the reaction
H2(g) + F2(g) --> 2HF(g)
 H = sum of the energy to break bonds (positive) + the sum of the energy to make bonds (negative).
H = sum of the energy to break bonds (positive) + the sum of the energy to make bonds (negative).
Then ![]() H = sum(D(bonds broken))
- sum(D(bonds formed))
H = sum(D(bonds broken))
- sum(D(bonds formed))
So for this example
![]() H = DH-H + DF-F
-2DH-F
H = DH-H + DF-F
-2DH-F
or ![]() H = 1mole*432 kJ/mol + 1mole*154
kJ/mol - 2moles*(565 kJ/mol) = -544 kJ
H = 1mole*432 kJ/mol + 1mole*154
kJ/mol - 2moles*(565 kJ/mol) = -544 kJ
Experimentally the answer for ![]() Ho
is -542 kJ so we estimated this pretty well. However the estimate is not usually
that good.
Ho
is -542 kJ so we estimated this pretty well. However the estimate is not usually
that good.
for another example:
CH4(g) + 2Cl2(g) + 2 F2(g) -->
CF2Cl2(g) + 2 HF(g) +2 HCl(g)
![]() H = 4DC-H + 2DCl-Cl
+ 2DF-F
H = 4DC-H + 2DCl-Cl
+ 2DF-F
- 2DC-F -2DC-Cl -2DH-F -2DH-Cl
= 4(413) + 2(239) + 2(154) - 2(485) - 2(339) - 2(565) - 2(427) = -1194 kJ
Give the homework a try now on these types of problems.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |
