I. Ionic Bond
This bond is formed by two ions coming together to form the ionic bond. Remember that there is really no such thing as an ionic molecule. The ions are arranged in a three-dimensional lattice rather than as single molecules. However, as when we were dealing with ionic reactions, we will treat the ionic compound as though it were a single molecule. Since this type of bond is between two ions, this is a pure ionic interaction and we can calculate the energy of the bond by Coulomb's law:
E = 2.31 x 10-19Jnm (q1q2/r)
Where q1 and q2 are the charges of the ions and r is the distance between the two ions. For NaCl, r = 0.276 nm and the charges are +1 and -1. Making these substitutions gives us the energy of the bond as
E = -8.37 x 10-19J or -5.04 x 105 J/mol or
E = -504 kJ/mol
This is a rather large number as we should expect for the ionic compounds all have high melting points.
II. Covalent Bond
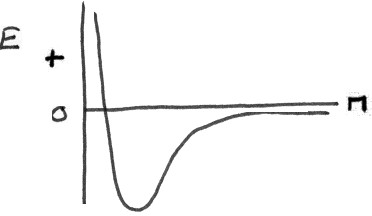
This bonding is very different than the ionic bonding. Now we do not have charged atoms forming bonds. An example will be the combination of two hydrogen atoms to form the hydrogen molecule. Imagine the two atoms separated by 100 yards and they start
coming together. As they approach each other closer, the electrons of the atoms will start spending more time in between the two nuclei and at some point (0.074 nm separation) they will form the most stable (the lowest energy) configuration. This can
be demonstrated on the following diagram where the energy is plotted vs the distance between the two hydrogen atoms.

III. Polar Covalent Bond
This final type bond is a covalent bond, meaning that there is "sharing" of electrons between the two atoms making up the bond, but the sharing is not equal. One of the atoms has a larger share of the electron density than the other. In H-F, the F has
a partial negative charge and the H has a partial positive charge. In H2O, the oxygen has a partial negative charge and the H has a partial positive charge. The bond then is slightly polar, meaning that there is an unsymmetrical arrangement
of charges. So why does F attract more electron density to it than H does? The reason is another inate ability of atoms. Remember that electron affinity is the ability of an atom to attract electron density to itself as a free atom. This new concept
is called electronegativity and is the tendency of atoms to attract electron density to itself when it is involved in bonding with another atom. The electronegativity is
usually given the symbol 
The Pauling scale of electronegativity is probably the most used scale and it is the one which is in your book. On this scale H has a  value of 2.1 and F has the value 4.0. F is the most electronegative element in the periodic table. So what does this
have to do with the polarity of a bond? Good question. Now we define another variable given the symbol
value of 2.1 and F has the value 4.0. F is the most electronegative element in the periodic table. So what does this
have to do with the polarity of a bond? Good question. Now we define another variable given the symbol  , which is the difference in electronegativity
values of the two atoms involved in the bond. The larger the
, which is the difference in electronegativity
values of the two atoms involved in the bond. The larger the  value, the greater the polarity of the bond. For example:
value, the greater the polarity of the bond. For example:
| Bond | H---H | S---H | Cl---H | O---H | F---H |
 | 2.1 2.1 | 2.5 2.1 | 3.0 2.1 | 3.5 2.1 | 4.0 2.1 |
 | 0 | 0.4 | 0.9 | 1.4 | 1.9 |
As you see the  increasing, the polarity of the bonds is increasing and the H-F bond is the most polar covalent bond.
increasing, the polarity of the bonds is increasing and the H-F bond is the most polar covalent bond.
Can a molecule containing polar bonds be a nonpolar molecule? Good question. If a molecule has a separtion of positive and negative charges in an unsymmetrical way then the molecule has a dipole moment and is said to be polar, like HF and water (water is not a linear molecule). the bonds in these molecules are also polar. But a molecule can have polar bonds and be a nonpolar molecule like carbon dioxide which is linear. Carbon dioxide looks like:
so that the partial negative charges on each oxygen are symmetrical about the partial negative charge on the carbon atom and the result is a nonpolar molecule. However the delta for the C-O bond is 1.0 so the bonds are polar.
Now consider water:
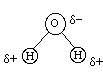
Water is a non-linear molecule and each bond is polar. So water is a polar molecule with polar bonds.
At this point I want to introduce another concept called the "hydrogen bond". This is an intermolecular force, not an intramolecular force so it involves bonding between two different molecules. An example of hydrogen bonding is between two water molecules:
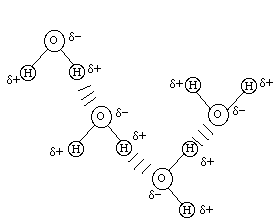
Here you see 4 water molecules held together by hydrogen bonding between the oxygen atom with it's partial negative charge and the hydrogen atom with it's partial positive charge. Such a bond is not a chemical bond but it is of great importance in dealing with many topics in applied chemistry in chemistry, biology, and other fields. This bond only occurs when a hydrogen atom is directly chemically bonded to a very electronegative atom (F,O,N,Cl) for in such cases the hydrogen atom will have a strong partial positive charge and the very electronegative atom will have a strong partial negative charge. Thus each molecule has strong partial charges which can be attracted to each other to form the hydrogen bond between the two (or more) molecules.
 Here is a site on bonding and polarity
Here is a site on bonding and polarity
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |
