 Here is a good site for a more detailed development of the Bohr theory of the atom. This site also has two
problems for you to practice.
Here is a good site for a more detailed development of the Bohr theory of the atom. This site also has two
problems for you to practice.
Niels Bohr proposed a model for the hydrogen atom that explained the spectrum of the hydrogen atom. The Bohr model was based on the following assumptions.
 Here is a good site for a more detailed development of the Bohr theory of the atom. This site also has two
problems for you to practice.
Here is a good site for a more detailed development of the Bohr theory of the atom. This site also has two
problems for you to practice.
Using the above assumptions, the energy of a hydrogen atom in level n was found to be
E = -RH/n2 where RH = 2.179 x 10-18
We can then calculate the change in energy when an electron changes from n=3 to n=2
![]() E = E3 - E2
= (--RH/32) - (--RH/22) or
E = E3 - E2
= (--RH/32) - (--RH/22) or
 E = -RH(1/22 - 1/32)
which also must be equal to h*
E = -RH(1/22 - 1/32)
which also must be equal to h*![]() .
.
Solving for 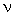 gives
gives
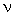 =
=  E/h = RH/h * (1/22 - 1/32) which gives
E/h = RH/h * (1/22 - 1/32) which gives
RH/h = 2.179 c 10-18J/6.626 x 10-34Js or
RH/h = 3.289 x 1015s-1
This is within experimental error of the constant in the Balmer equation for a hydrogen transition series (3.2881 x 1015s-1) which really made everyone take notice.
 Go here to obtain further insight into the Bohr model of the atom. Be sure to click on "Next" at the bottom of this site to
continue with the explanations.
Go here to obtain further insight into the Bohr model of the atom. Be sure to click on "Next" at the bottom of this site to
continue with the explanations.
However, the Bohr model
People tried modifying the theory by allowing the electrons to have elliptical orbits and other changes, but when you have a bad model you need to start over and that is what we will do next.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |
