You will now learn about a more general theory for acids and bases that explains acid-base behavior regardless of the solvent.
Synopsis
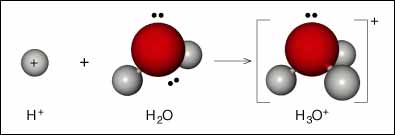
G. N. Lewis developed a theory of acids and bases that is based upon the sharing of electron pairs. A Lewis acid is a substance that can accept a pair of electrons from another atom to form a new bond. A Lewis base is a substance that can donate a pair of electrons to another atom to form a new bond. A simple example is the formation of the hydronium ion from a proton (no electrons) and water (has electron pairs to donate):

The product is called the adduct or complex. When one substance donates all the electrons for the bond the bond is called the coordinate covalent bond (page 722). In this example, H+ is the Lewis acid and H2O is the Lewis base.
All metal cations are potential Lewis acids because their positive charge will readily attract electron pairs and they all have at least on empty orbital. The hydroxide ion is an excellent Lewis base and so it will bind readily to metal cations to give metal hydroxides. Some of these metal hydroxides are amphoteric. An amphoteric metal hydroxide can behave as a Bronsted base and react with a Bronsted acid, or it can behave as a Lewis acid and react with a Lewis base.
The Lewis acid-base model also accounts for the fact that oxides of nonmetals behave as acids. An example is carbon dioxide, which has acid behavior that can be understood in this model. Because the oxygen atom in CO2 is more electronegative than the carbon atom, the carbon atom will have a slight positive charge that will be attractive to the OH- group. The CO2 molecule then acts as a Lewis acid accepting the donated electrons form the hydroxyl group.
Review Questions

Web Author: Dr. Leon L. Combs
Copyright ©2000 by Dr. Leon L. Combs - ALL RIGHTS RESERVED