Now you will learn the eight types of intermolecular forces and be able to relate these abstract concepts to physical properties of molecular systems.
Synopsis
There are seven intermolecular forces in the attractive category, and one type of repulsive intermolecular force. If we did not consider the repulsive force then our model would predict that all matter should collapse to a point in the universe!
The first type of attractive intermolecular force, ion-ion interaction, is rather simple to discuss. An example of ion-ion interaction would be the interaction between a Na+ ion and a Cl- ion in a water solution of NaCl. The energy of interaction between the two ions is easily understood in terms of Coulomb's law:
E(r) = Cq1q2/d
with q1 and q2 being the charges on the ions, d is the distance between the ions, and C is a constant for units.
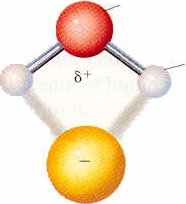
The second type of attractive intermolecular force, ion-dipole interaction, is also rather easy to conceptualize. We can again consider our water solution of NaCl, but now focus on the interaction between the water molecules and the ions in solution:

The energy of interaction can also be calculated using Coulomb's law and this energy is called the solvation energy or the energy of hydration because the ions are hydrated. This energy is quite large and, because it decreases as d increases, it will decrease as the size of the ion increases (such as Li+ --> Cs+). Because the H+ ion is so small, it has the largest hydration energy (-1090 kJ/mol). We commonly represent the hydrated H+ ion as H3O+. Salts can also become hydrated by an attraction between the charge of the positive ion of the salt and the water dipole such as BaCl2 * 2 H2O.
The third type of attractive intermolecular force is the dipole-dipole interaction. Returning to our salt/water solution, this type of interaction is represented by the water-water interactions due to the attractions among the permanent dipole moments of the water molecules. Quantitatively the energy of this interaction is a bit more complicated to describe due to the many possible mutual orientations of the water dipoles. However, examining the differences in boiling points between polar and non-polar molecules of approximately the same size easily verifies the effect of the dipole-dipole interactions. This effect is demonstrated in Table 13.2 of your text. The process of boiling involves transforming the molecules from the liquid state to the gaseous state. This transformation is initiated by putting enough energy into the liquid to break the intermolecular forces holding the molecules together in the liquid state.
The fourth type of attractive intermolecular force is the hydrogen bond. This bond is an intermolecular bonding - generally not intramolecular bonding - between a hydrogen atom intramolecularly bonded to a very electronegative element (usually F, O, or N) and a very electronegative element on another molecule. An example is the hydrogen bond interaction between different water molecules:
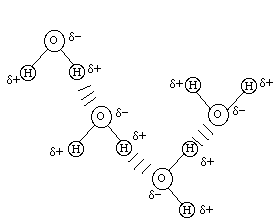
There can be more than one attractive intermolecular hydrogen bond between two molecules such as between two acetic acid molecules (CH3COOH):

The strongest hydrogen bond would be that between two HF molecules and this bond is so strong that it even exists in the vapor state. Hydrogen bonding is very important in the chemistry of biological molecules, including the DNA structure that is stabilized in its helical coil by hydrogen bonds (in this case these are intramolecular bonds).
The fifth type of intermolecular force is the ion-induced dipole force. An induced dipole occurs when a charge near a molecule causes the electron distribution of the molecule to become distorted from its original distribution. The molecule may or may not have a permanent dipole moment. A permanent dipole moment exists in a molecule that has charges distributed in an unsymmetrical manner, such as H2O. Molecules such as O2 or benzene do not have a permanent dipole moment and a charge can come near and induce an unsymmetrical charge distribution with a resulting instantaneous dipole moment. Once the instantaneous dipole moment is induced, there can then be an ion-induced dipole interaction. The process of inducing a dipole is called polarization and different atoms and molecules have different polarizabilities (ability to be polarized)
The sixth type of intermolecular force is the dipole-induced dipole force. A dipole rather than a charge now induces the instantaneous dipole moment. An example is the attraction that is known to exist between O2 and water. Oxygen is slightly soluble in water, and for that solubility to exist there must be an attraction between water and oxygen. Oxygen is a symmetrical molecule so it does not have a permanent dipole moment. However, the water dipole can cause the charges on oxygen to become unsymmetrical with a resulting instantaneous dipole moment:

The seventh type of attractive intermolecular force is the induced dipole - induced dipole interaction. This type of interaction is also called dispersion interaction or London dispersion interaction. This type of interaction can occur between two nonpolar molecules such as between two argon atoms. This dispersion interaction can be demonstrated by the following:

The atoms begin spherically symmetrical, but their interactions can induce an instantaneous dipole in each atom and then the induced dipole - induced dipole interaction will momentarily stabilize the pair. Such stabilization means that the species can exist as a liquid at STP (e.g. Br2) instead of as a gas, such as Cl2. The interactions in argon are not strong enough to stabilize argon as a liquid at STP
The second category of intermolecular forces is the repulsive intermolecular forces, and there is only one type of these: van der Waals repulsion. These forces occur when the atoms or molecules come very close to each other and the repulsion of like charges becomes strong. These forces vary as 1/d12 and you can see from this mathematical form that when d gets very small this becomes very large. This is the force that stops all matter from coalescing to a single point in the universe. An equation which combines the attractive and repulsive forces is the Lennard-Jones "6-12" potential which writes the potential energy of interaction as
U(d) = - A/d6 + B/d12
Intermolecular forces are the factors influencing the observed solubilities. A common statement is that "like dissolves like", meaning that if two molecules are of the same type (polar or nonpolar) they are more likely to be soluble in each other. For example, water and methanol are both polar so we would predict that they would be soluble in each other.
Review Questions
1.) Plot the Lennard-Jones "6-12" potential (U(d) above vs d) to see how rapidly the curve rises as d becomes very small.
2.) Which should boil at the higher temperature, ethanol or dimethyl ether? Explain.
3.) State all of the intermolecular forces present in a NaCl - water solution. Don't just give a type, also sketch the specifics.
4.) Would you expect benzene to be soluble in water? Explain.
5.) Go to this site for a fuller discussion of intermolecular forces. It is a little deep for this level course, but some of it will be illuminating to you

Web Author: Dr. Leon L. Combs
Copyright ©2000 by Dr. Leon L. Combs - ALL RIGHTS RESERVED