Temperature and Density
Temperature
First you might ask "What is temperature?". Good question. Temperature is a measure of the kinetic energy and thus the motion of the molecules (KE=0.5 * m * v2). We will pursue this insight later as we continue in our pursuit to understand
why water boils at a higher temperature than ethanol.
As a resident of the U.S.A., you are used to hearing temperature expressed in degrees Fahrenheit. Most of the rest of the ordinary world expresses temperature in degrees Celsius. We weird scientists prefer to use Kelvin as the measure of temperature.
Let's indicate the three thermometers with the following picture:
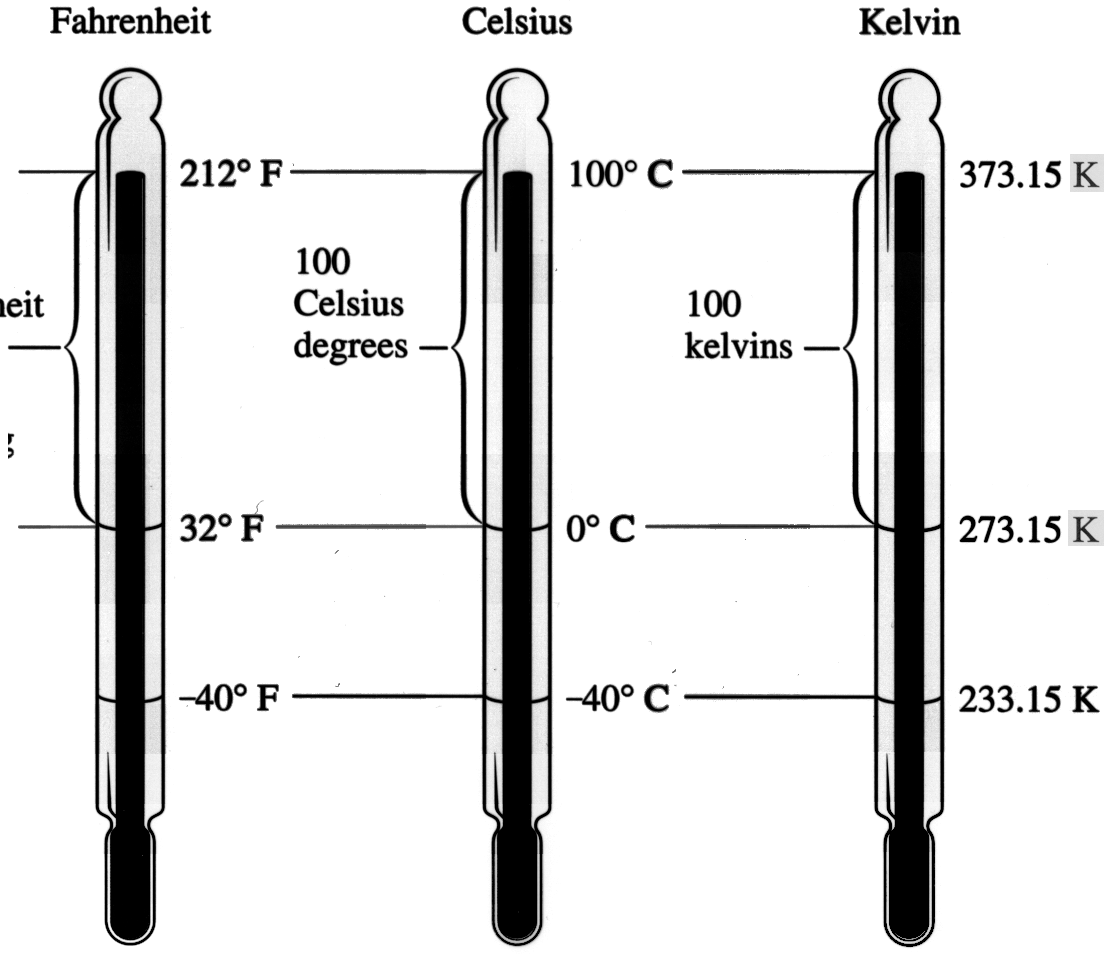 The left thermometer is Fahrenheit which did not quite copy. From this picture you can see that the length from the freezing point of water to the
boiling point of water for the Celsius and the Kelvin thermometers is the same as is the degrees (100). So conversion from degrees Celsius to Kelvin is easy: just add 273.15 to the degree Celsius.
The left thermometer is Fahrenheit which did not quite copy. From this picture you can see that the length from the freezing point of water to the
boiling point of water for the Celsius and the Kelvin thermometers is the same as is the degrees (100). So conversion from degrees Celsius to Kelvin is easy: just add 273.15 to the degree Celsius.
However converting between F and C is more difficult since they have different zero points. We see that 180 degrees on the F scale is the same as 100 degrees on the C scale so we can set up a unit factor of 1800F/1000C =
90F/50C. So to convert C to F we need to calculate:
TF = TC*90F/50C + 320F
And to convert F to C we would need to calculate
TC = (TF - 320F)*50C/90F
Another concept which we need to know is
Density
Density is determined by dividing the mass of the substance by its volume. Some common liquids and their densities are the following (don't memorize):
Compound Density (g/cm3)
ethanol(l) 0.789
water(l) 0.9982
oxygen(g) 0.00133
salt(s) 2.16
 Now you are ready again to go to THIS SITE and practice, practice, practice.
Now you are ready again to go to THIS SITE and practice, practice, practice.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |

Web Author: Dr. Leon L. Combs
Copyright ©1999 by Dr. Leon L. Combs - ALL RIGHTS RESERVED
 The left thermometer is Fahrenheit which did not quite copy. From this picture you can see that the length from the freezing point of water to the
boiling point of water for the Celsius and the Kelvin thermometers is the same as is the degrees (100). So conversion from degrees Celsius to Kelvin is easy: just add 273.15 to the degree Celsius.
The left thermometer is Fahrenheit which did not quite copy. From this picture you can see that the length from the freezing point of water to the
boiling point of water for the Celsius and the Kelvin thermometers is the same as is the degrees (100). So conversion from degrees Celsius to Kelvin is easy: just add 273.15 to the degree Celsius.
