
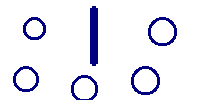
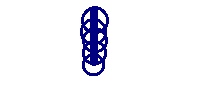
A number with no units is meaningless. If I say that I weigh 90.8 how much information does that convey to you? None, right? However if I put a unit on that and say that I weigh 90.8 kgm, then that is meaningful, although perhaps not a whole lot of help to people who are used to pounds and inches (English system). Another system used by most of the rest of the world is the metric system. However even agreeing to use the metric system is not complete enough.
It is important that everyone throughout the world use the same units of measurement so that we can effectively communicate. Since 1960 scientists have used the SI system of measurement, but not totally as we will see as we progress through the semester. The SI stands for the International System (le Systeme International, in French).
The Fundamental SI Units are as listed below:
Physical Quantity Name of Unit Abbreviation Mass kilogram kg Length meter m Time second t Temperature Kelvin K Electric Current ampere a Amount of substance mole mol Luminous intensity candela cd
Derived SI units come from combinations of the above such as the following:
area square meter m2 volume cubic meter m3 velocity meter per second m s-1 acceleration meter per second squared m s-2 density kilogram per cubic meter kg m-3 molar mass kilogram per mol kg mol-1 molar volume cubic meter per mole m3 mol-1 molar concentration mole per cubic meter mol m-3
The prefixes used with these units are given below and in your book (Table 1-2) and you must memorize them today.
Prefix Symbol Exponential Notation exa E 1018 penta P 1015 tera T 1012 giga G 109 mega M 106 kilo k 103 hecto h 102 deka da 101 deci d 10-1 centi c 10-1 milli m 10-3 micro mc or µ 10-6 nano n 10-9 pico p 10-12 femto f 10-15 atto a 10-18
When you have seen one measurement you have seen them all is certainly not true. There are three concepts involved:
If we want to measure this line: ______________________________
we could get several different answers depending upon the measuring device. One device might give 3.5 inches by one person with measuring device A and 3.56 by another person with measuring device B. However when they swap devices, they might not get the same results as recorded initially with A and B. So all three concepts are involved. The slide below neatly shows the difference between accuracy and precision.

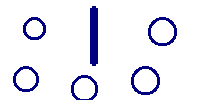
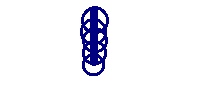
Imagine that the above represents the results of a "ring toss" game. The first image is clearly very precise, but not accurate. The second has rings all over the place and hence is neither precise nor accurate. The third is "just right" --
precisely accurate
Now suppose that person 1 measures the above line with device A and person 2 measures the length of this line __________with device B. What is the sum of the lengths of the two lines? First we must be certain that the accuracy of A is really only in the tenths of inches and the accuracy of B is really to the hundredths of inches. Assuming that such is a fact, then how do we determine the sum? Well now we are into a new topic: significant figures.
There are some rules to follow to allow you to get the significant figures done correctly. I summarize them as follows:
![]() Now you might want to go to This Tutoring Site for more help with significant figures.
Now you might want to go to This Tutoring Site for more help with significant figures.
Often we have to convert from one system of units to another. For example, you may be wondering how much I really weigh since I told you earlier that I weigh 90.8 kgm. The method of unit conversion is called either the unit factor method or dimensional analysis. In order to make conversions we must know some conversion factors. Two of the more useful are that there are 2.54 cm in one inch and that there are 453.6 gms in one pound. Let's consider the gram/pound conversion.
We have the equality:
453.6 gms = 1 pound
So divide both sides by 453.6 gms and we have
453.6 gms/453.6 gms = 1 = 1 pound/453.6gms
So since 1 pound divided by 453.6gms is equal to one, multiplying by that ratio will not change the value of the numerical value of the number -- just the unit. So let's give it a try.
90.8kgm x 1000gm/kgm = 90800 gms where we used another definition ratio (1000gm/1kgm). Now use the pound conversion:
90800gms x 1 pound/453.6 gms = 200.2 pounds. But what about sig figs? We only have 3 sig figs in the given number of 90.8 kgm so we say that I weigh 200. pounds where I put a "." after the number 200 which indicates that all three digits are significant. Alternatively we could say that I weigh 2.00 x 102 pounds.
![]() Now you might want to go to This Tutoring Site for help in unit conversions.
Now you might want to go to This Tutoring Site for help in unit conversions.
![]() Now you are ready to go to THIS WEB SITE to practice doing all of the above. In learning this type of material it is crucial to practice.
Now you are ready to go to THIS WEB SITE to practice doing all of the above. In learning this type of material it is crucial to practice.
| Now take a practice quiz to help you understand if you understand the basic concepts. |
| You must use your real name when it asks for a name. |
| The test will only submit when you have answers all of the questions correctly. |
| If you are not taking this course for credit please do not answer all the questions correctly for I don't want to be flooded with email answers to the tests. |

Web Author: Dr. Leon L. Combs
Copyright ©1999 by Dr. Leon L. Combs - ALL RIGHTS RESERVED