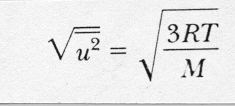 ...eq 9
...eq 9
This section introduces you to a model for understanding the macromolecular properties of this chapter.
Synopsis
This theory is based upon ideal gas behavior with the following assumptions:
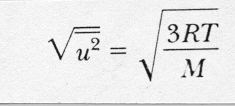 ...eq 9
...eq 9
This equation for the rms velocity is also sometimes called "Maxwell's equation" although Maxwell developed many other equations that bear his name (e.g. Maxwell's equations of thermodynamics and Maxwell's equations of electrodynamics) so we should perhaps call it "Maxwell's velocity equation". The following figure shows the Maxwell-Boltzmann velocity distribution of oxygen molecules at different temperatures.
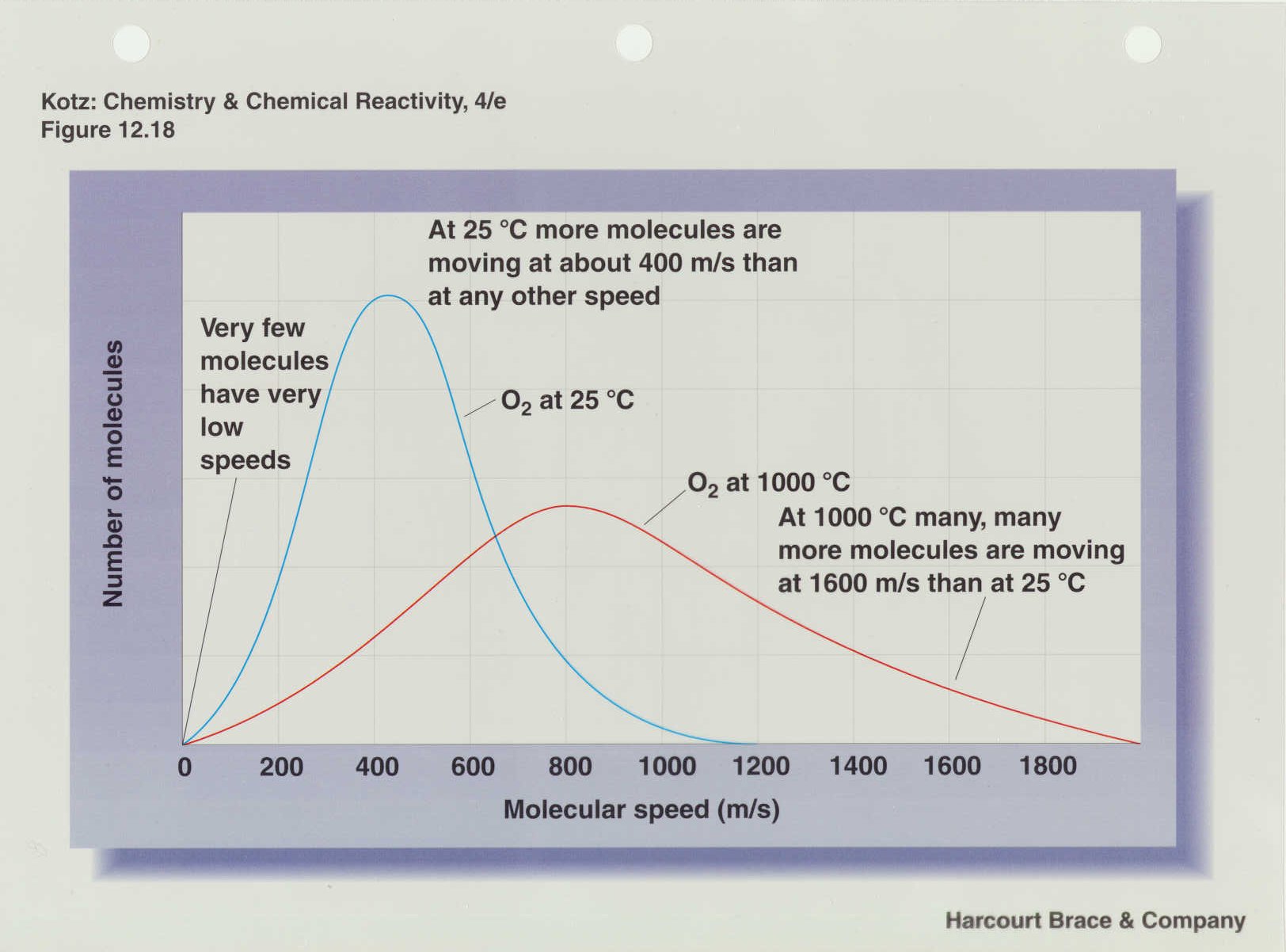
We can also determine the "most-probable velocity" using this model and that velocity will correlate with the maximum of the distribution (a little over 200 m/s at 25 oC and a little over 800 m/s at 1000 oC). The equation for this velocity is ump =
(2RT/M).5. Also note that the curves in the figure above are not symmetrical about the maximum. That means that the average velocity will be another type of velocity and it will be where the areas under the curve on each side of that velocity are
equal. The average velocity is given by uav = (8RT/pi*M).5. Figure 12.19 in your text shows the Maxwell-Boltzmann velocity distribution for different gases at the same temperature. It is important for you to convert some of these velocities into the
more common units in America so that you gain an appreciation for the rate at which events occur on the molecular level.
Review Questions

Web Author: Dr. Leon L. Combs
Copyright ©2000 by Dr. Leon L. Combs - ALL RIGHTS RESERVED