You will learn the six common units for pressure, and you will obtain practice in converting among the units.
Synopsis
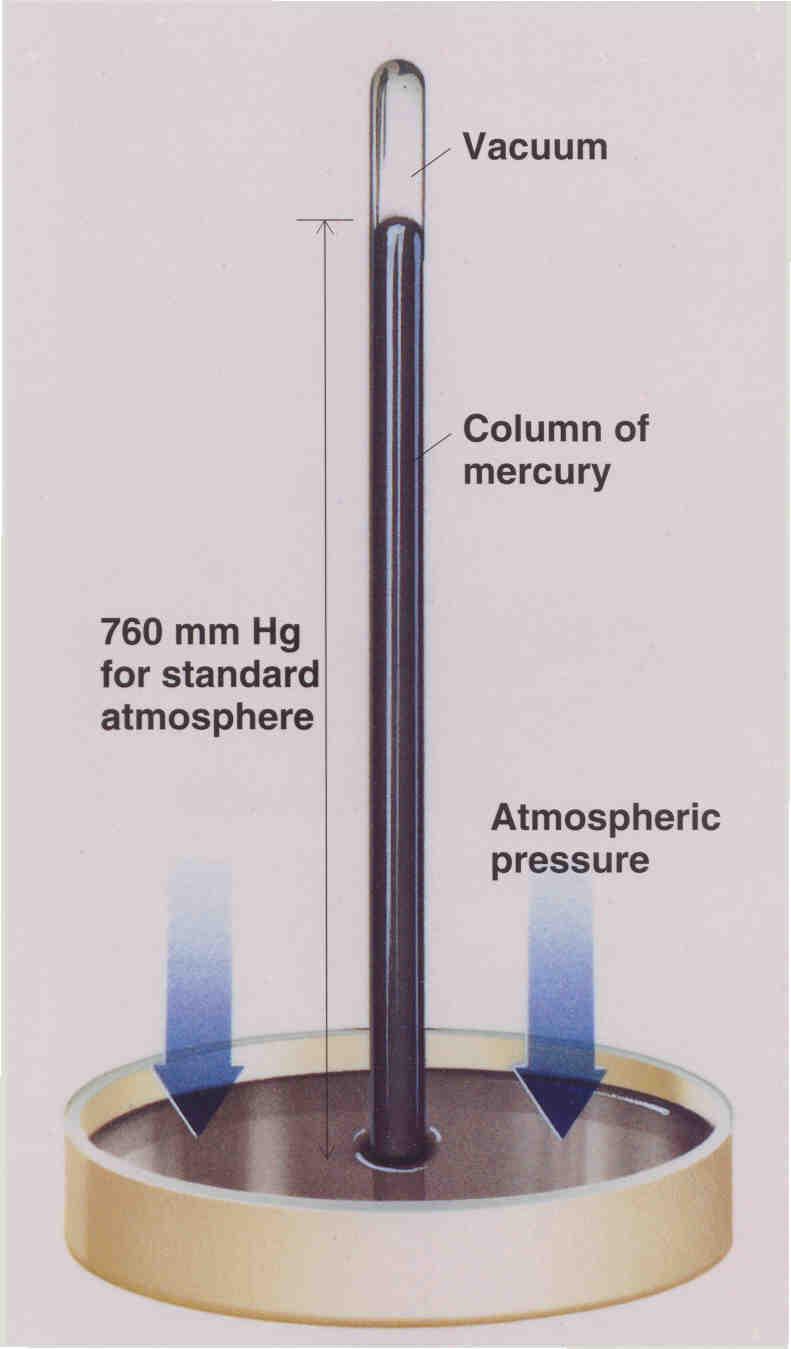
You know that pressure is force per unit area, so you would think that the pressure units would then have the units of newtons/meter2. This is very logical, and is indeed the SI unit of pressure. This unit of newtons/meter2 is given the name pascal in honor of Blaise Pacal, and the symbol Pa. Pressures are also sometimes reported in units of bar where 1 bar = 100,000 Pa. Another common unit is millimeters of Hg because of the use of a mercury barometer to measure the atmospheric pressure:

A millimeter of Hg is also called torr in honor of Torricelli who invented this barometer. Another unit is atm where 1 atm is defined as 760 torr exactly. And we must not forget the engineers who still use pounds and inches, which gives us another unit for pressure -- the psi (pounds/inch2). So we then have the following interrelationships among these pressure definitions
1 atm = 760 torr = 760 mmHg = 1.013 bar = 101.325 kPa = 14.7 lb/in2
Pressures expressed in Pa are quite small so units of kPa are usually used.
Review Questions
It is very important to practice converting between these units before you proceed with your study.

Web Author: Dr. Leon L. Combs
Copyright ©2000 by Dr. Leon L. Combs - ALL RIGHTS RESERVED